Transfusion medicine
Transfusion medicine handbook
The Transfusion Medicine Handbook is designed to assist hospital staff and other health professionals in modern Transfusion Medicine Practice.
5. Fractionated Products
5.4.6 ZOSTER IMMUNOGLOBULIN-VF
Zoster Immunoglobulin-VF is a sterile, preservative-free solution containing not less than 200 IU/vial varicella-zoster antibody. Plasma for Zoster Immunoglobulin-VF is obtained from blood donors who have recently recovered from shingles or chickenpox. Donations are selected on the basis that they contain high levels of antibodies against Herpesvirus varicellae. Zoster Immunoglobulin-VF is intended for intramuscular injection [8].
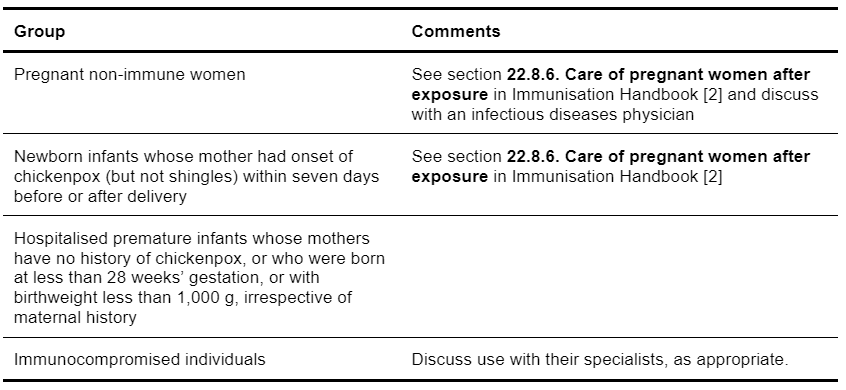
The decision whether to offer Zoster Immunoglobulin-VF depends on:
- The likelihood that the exposed person is susceptible to varicella
- The probability that a given exposure to varicella will result in infection
- The likelihood that complications would develop if the person exposed is infected [2].
Contact (exposure) can be classified as follows:
- Household contact – infection is very likely to occur in a susceptible individual living with an infected contact
- Playmate contact – more than one hour of play indoors with infected individual
- Newborn infant contact – when the mother of a newborn infant develops chickenpox (but not shingles) from seven days before to seven days after delivery
- Hospital contact – individuals in the same two-bed room or have face-to-face contact for longer than five minutes [2].
Indications for Use
Zoster Immunoglobulin-VF is indicated for prophylaxis against varicella in patients where exposure has occurred and susceptibility is likely. See Table 5.17 for specific groups.
Table 5.17: Use of Zoster Immunoglobulin-VF for prophylaxis against varicella in patients where exposure has occurred and susceptibility is likely.
The Starship Child Health Clinical Guideline covering Varicella zoster (chicken pox) post exposure prophylaxis [9] and the National Child Cancer Network guideline for Immunisation of Children During and After Cancer Therapy [10] provide additional specific detail on the use of zoster immunoglobulin in paediatric post-exposure prophylaxis. Consultation with a specialist paediatrician or NZBS Transfusion Medicine Specialist/Medical Officer is recommended prior to prescription.
Varicella-zoster immunoglobulin is of no value in the treatment of established varicella or zoster infection. High levels of circulating antibody do not prevent dissemination of infection.
Dosage and Administration
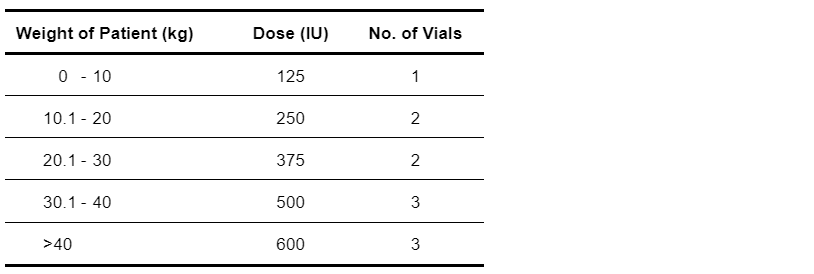
The required dose is 125 IU per 10 kg bodyweight and rounded up to the nearest 200 IU to a maximum of 600 IU (see Table 5.18).
Table 5.18: Weight-Based Dosing Schedule for Zoster Immunoglobulin-VF [8]
An intravenous preparation of Varicella zoster immunoglobulin is appropriate when the patient has a significant haemostatic defect which may cause bleeding following intramuscular injection. In New Zealand, Intragam P may provide Varicella zoster immunoglobulin at an estimated level of 10 IU/mL. Privigen NZ can also be used with an estimated level of 16.67 IU/mL, (note that Intragam P is a 6% solution while Privigen NZ is a 10% solution). Consultation with an NZBS Transfusion Medicine Specialist/ Medical Officer is recommended prior to prescription.