Transfusion medicine
Transfusion medicine handbook
The Transfusion Medicine Handbook is designed to assist hospital staff and other health professionals in modern Transfusion Medicine Practice.
5. Fractionated Products
5.4.4 TETANUS IMMUNOGLOBULIN-VF
Tetanus Immunoglobulin-VF is a sterile, preservative-free, pasteurised solution with a tetanus antitoxin activity of not less than 100 IU/mL. Donations used in the preparation of tetanus immunoglobulin are selected on the basis that they contain high levels of specific antibodies against the toxin of Clostridium tetani. Tetanus Immunoglobulin-VF is intended for intramuscular injection [7].
Tetanus Immunoglobulin-VF is indicated for:
- Passive protection of individuals who have sustained a tetanus-prone wound and who have either not been actively immunised against tetanus or whose immunisation history is doubtful
- Passive protection of fully immunised individuals with a tetanus-prone wound if more than 10 years have elapsed since the last dose of tetanus toxoid vaccine
In both of the above instances, active immunisation with tetanus vaccine should be commenced at the same time. Although tetanus immunoglobulin and vaccine can be given at the same time, they should be administered in opposite limbs, using separate syringes.
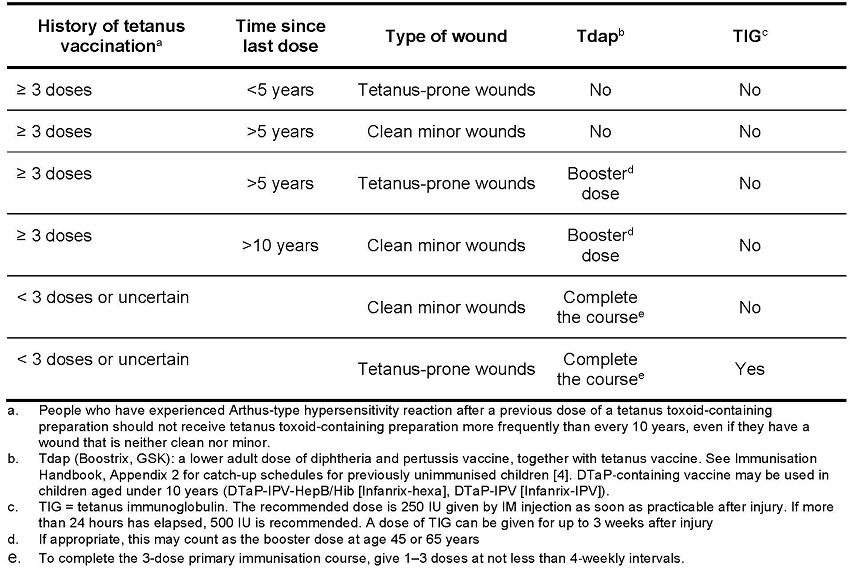
Table 5.12: Guide to Tetanus Prophylaxis in Wound Management [5]
Dosage and Administration
Good medical care is essential in the prevention of tetanus from fresh wounds. Thorough cleansing and removal of all foreign and necrotic material from the site of injury is important.
The minimum routine prophylactic dose of Tetanus Immunoglobulin-VF for adults or children is 250 IU given slowly by deep intramuscular injection. The dose should be doubled to 500 IU if the wound is grossly contaminated or if more than 24 hours have elapsed since injury or if there is a risk of heavy contamination or following burns. See Table 5.12 for guidance on tetanus prophylaxis and wound management.
An intravenous preparation of tetanus antitoxin is appropriate for patients where large doses are indicated (i.e., treatment of tetanus), or when the patient has a significant haemostatic defect which may cause bleeding following intramuscular injection. In New Zealand, Privigen NZ may be used to provide intravenous tetanus immunoglobulin at a dose of 300 mg/kg. Consultation with an NZBS Transfusion Medicine Specialist/ Medical Officer is recommended prior to prescription.
Treatment of Suspected or Confirmed Clinical Tetanus
For the treatment of suspected or confirmed clinical tetanus it is recommended that a single dose of Privigen NZ at 300 mg/kg be given, which is compatible with CDC advice [9]. However, it is acknowledged that the optimal dose, which may be as low as 500 IU, is not yet defined.
