Transfusion medicine
Transfusion medicine handbook
The Transfusion Medicine Handbook is designed to assist hospital staff and other health professionals in modern Transfusion Medicine Practice.
6. Special Circumstances
6.17 Special Requirements
Neonates less than 1500 g in weight or immunosuppressed for other reasons should be given ‘CMV safer’ blood components as an added precaution. The use of either pre-storage leucodepletion or selection of CMV-antibody negative blood components significantly reduces the risk of CMV transmission and CMV disease in susceptible recipients. However, neither method alone or in combination can completely avoid transmission from the occasional donor with CMV viraemia in the "window" period prior to the development of antibodies following acute infection or when reactivation of latent infection occurs.
In some situations, irradiated blood components may be indicated for neonatal transfusions. Irradiation removes the small risk of transfusion-associated graft-vs-host disease (TA-GvHD) in at-risk patients by inactivating donor lymphocytes.
When there is clinical suspicion of a congenital cellular immunodeficiency state such as severe combined immunodeficiency, DiGeorge syndrome, Wiskott-Aldrich syndrome, ataxia telangiectasia or partial deletion of chromosome 22 (del 22q11.2), use of irradiated components is mandatory. Features associated with immunodeficiency states include cardiac defects, dysmorphic features, craniofacial abnormalities, hypocalcaemia and lymphopenia.
Irradiated components are not indicated for ‘top-up’ transfusions of premature or term infants unless there has been a previous IUT, in which case irradiated components should be administered until 6 months after the 40 week gestation date.
Irradiated components may also be appropriate for extreme low body weight neonates less than 28 weeks who are at increased risk of TA-GVHD due to physiological immune incompetence. Evidence suggests that the risk is greatest in those < 900 g in body weight.
As a result of gamma irradiation red cells lose potassium during storage. While this is not normally a problem with small volume neonatal top-up transfusions, for larger volume and exchange transfusions irradiated red cells must be transfused within 24 hours of irradiation.
Use of Erythropoeitin
Erythropoietin (EPO) may be used to reduce transfusion requirements. Specialist advice on dose and frequency should be obtained.
Platelets
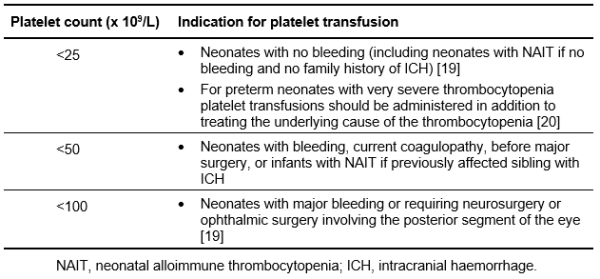
There is an increased risk of haemorrhage with thrombocytopenia. The risk is increased if sepsis or coagulopathy are present (see Table 6.14 for platelet thresholds for transfusion).
Table 6.14: Suggested thresholds of platelet count for neonatal platelet transfusion.
In a randomised controlled trial of preterm neonates with severe thrombocytopenia (platelet count <25 x 109/L), prophylactic platelet transfusions to maintain a platelet count threshold of 50 x 109/L led to a significantly higher rate of mortality or major bleeding within 28 days of randomisation than those transfused to maintain a platelet count threshold of 25 x 109/L, supporting a restrictive approach to prophylactic platelet transfusion [20].
The recommended volume for top-up transfusion is 10 mL/kg to a maximum of one neonatal unit. A single neonatal platelet unit will normally provide an acceptable platelet increment in children under 10 kg in body weight. At higher body weights, one single neonatal platelet unit per 10 kg of body weight followed by a post-transfusion platelet count and reassessment of the patient is recommended. If the plasma volume of the platelet concentrate is excessive, the blood bank may be asked in advance for advice regarding the need to remove plasma to a minimum volume of 10 - 15 mL per platelet concentrate.