Transfusion medicine
Transfusion medicine handbook
The Transfusion Medicine Handbook is designed to assist hospital staff and other health professionals in modern Transfusion Medicine Practice.
5. Fractionated Products
5.1.4 FEIBA NF (Factor VIII inhibitor bypassing fraction)
FEIBA NF is a sterile freeze-dried powder of human plasma fraction with factor VIII inhibitor bypassing activity. The potency of FEIBA NF is expressed in arbitrary units. One Unit of activity is defined as that amount of FEIBA NF that shortens the activated partial thromboplastin time (aPTT) of a high titre factor VIII inhibitor reference plasma to 50% of the blank value.
FEIBA NF is available as 20 mL vials containing 500 U and 1000 U factor VIII inhibitor bypassing activity for reconstitution and intravenous administration.
FEIBA NF is prepared by adsorption of coagulation factors from plasma onto an ion-exchange medium followed by selective elution. The manufacturing process of FEIBA NF contains dedicated steps including nanofiltration and vapour heat treatment to reduce the potential for viral transmission. The current procedures are effective for inactivation/removal of HIV, hepatitis B, and hepatitis C and may also have some effect on hepatitis A and parvovirus B19.
FEIBA NF is a concentrate of vitamin K-dependent factors in both zymogen and active form (factors II, IX, and X, mainly non-activated, and factor VII, mainly activated). The product contains approximately equal unit of factor VIII inhibitor bypassing activity and prothrombin complex factors. In addition, 1 - 6 units of factor VIII coagulant antigen (FVIII C:Ag) per mL are present. The preparation contains only traces of factors of the kinin generating system. It contains no heparin.
The process of coagulation involves activation of factor X to form Xa, which with cofactor Va catalyses the formation of thrombin from prothrombin. The production of sufficient quantities of Xa usually requires a complex of factors VIIIa and IXa. Patients (often those with haemophilia A or B) can acquire inhibitors to factor VIII or IX during the treatment with replacement therapy, which then prevents the formation of the complex that catalyses Xa production. FEIBA NF results in the generation of Xa and thrombin without the help of the factor VIIIa-IXa complex, thereby bypassing the inhibitory action of factor VIII (or factor IX) inhibitors.
Indications for Use
FEIBA NF is indicated for:
- Routine prophylaxis of bleeding episodes in haemophilia A and B patients with inhibitors, experiencing ≥ 12 bleeding episodes per year and refractory to increased dosing with, respectively, factor VIII and IX concentrates.
- Treatment of bleeding episodes and to cover surgical interventions in haemophilia A and B patients with factor VIII and IX inhibitors, respectively.
- Long term use, in combination with factor VIII concentrates, for immune tolerance induction to eliminate factor VIII inhibitors in patients with haemophilia A, so as to allow for regular treatment with factor VIII concentrates as in patients without inhibitors.
- Treatment of severe or life-threatening bleeding episodes in non-haemophiliacs with acquired inhibitors to factors VIII, XI and XII.
FEIBA NF may be used for treating non-haemophiliacs with acquired inhibitors to Factors VIII, XI and XII in case of severe or life-threatening haemorrhages.
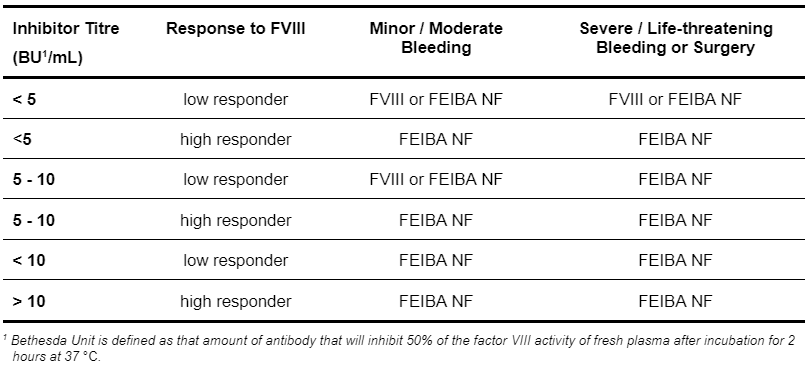
For guidelines for treatment of patients with FVIII inhibitors see following Table 5.4.
Table 5.4: Guideline for Treatment of Patients with Haemophilia A and Factor VIII Inhibitors
Dosage and Administration
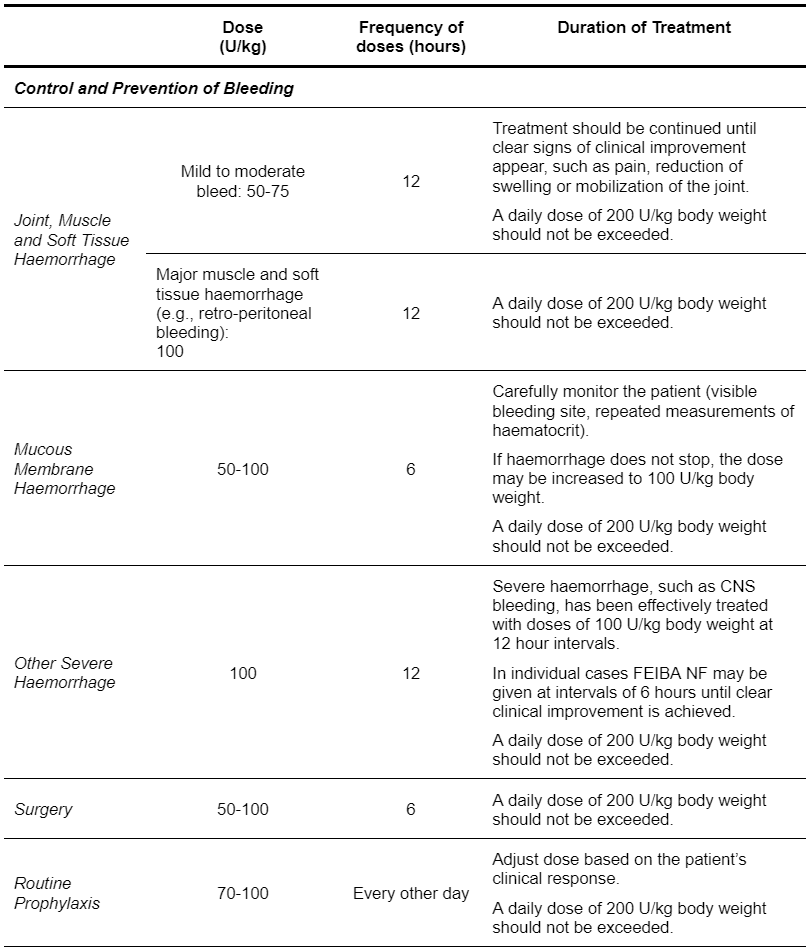
Dosage is independent of the patient's inhibitor titre. As a general rule, a dose of 50 - 100 U FEIBA NF per kilogram body weight is recommended. However, due to the risk of thrombosis, the total daily dose should not exceed 200 U/kg and, for any one dose, the infusion rate should not exceed 2 U/kg per minute. Due to varying response to treatment the following dosage recommendations are only guidelines.
Table 5.5: FEIBA NF Dosing Guideline
Contraindications
FEIBA NF is contraindicated in cardiac surgery involving cardiopulmonary bypass and procedures involving extracorporeal membrane oxygenation (ECMO) due to the high risk of thrombotic adverse events.
Precautions
- Allergic reactions
FEIBA NF has been associated with severe allergic and anaphylactoid reactions. - Anamnestic response
Administration of FEIBA NF to patients with inhibitors may result in an initial anamnestic rise in inhibitor levels. Clinical data suggest that the efficacy of FEIBA NF is not reduced. Inhibitor levels may decrease over time with continued administration of FEIBA NF. - Laboratory tests
In vitro tests such as aPTT, whole blood clotting time (WBCT) and thromboelastography (TEG) used to monitor efficacy may not correlate with clinical improvement. Attempts to normalise these values by increasing the dose of FEIBA NF are discouraged and may induce DIC through overdosage. - Passive anti-HBs transfer
Administration of FEIBA NF with a transitory rise of passively transferred hepatitis B surface antibodies may mislead the interpretation of positive serology test results. - Sodium
The maximum daily dose of FEIBA NF may contain > 200 mg sodium and this should be taken into consideration for patients on a sodium-restricted diet and/or with renal impairment. - Thrombosis
Patients should not receive single doses of FEIBA NF > 100 U/kg body weight or daily doses > 200 U/kg body weight as these may predispose venous and arterial thromboembolism, DIC, myocardial infarction or stroke. Patients receiving doses such as these should be monitored for the development of adverse events. The possible presence of risk factors for thromboembolism, even in patients with haemophilia, should always be considered.
Interactions with Other Medicines
The use of tranexamic acid, an antifibrinolytic agent, in combination with FEIBA NF is not recommended due to an increased risk of thrombotic events. If treatment with both is indicated the products should be administered at least 12 hours apart. Concomitant use with recombinant factor VIIa may potentially result in an adverse thrombotic event.