Transfusion medicine
Transfusion medicine handbook
The Transfusion Medicine Handbook is designed to assist hospital staff and other health professionals in modern Transfusion Medicine Practice.
5. Fractionated Products
5.1.1 BIOSTATE (Factor VIII)
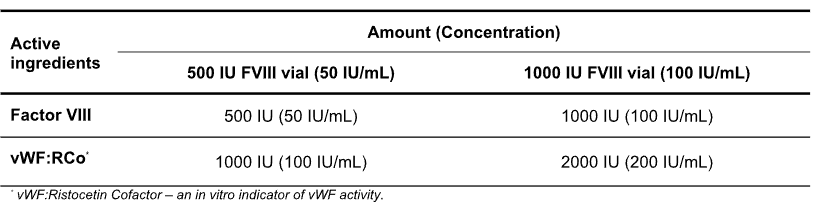
Biostate is a high purity, sterile, freeze-dried powder containing purified human coagulation factor VIII (FVIII) and human von Willebrand factor (vWF) complex. Biostate is available as vials of 500 IU and 1,000 IU factor VIII. The reconstituted product has a vWF to FVIII ratio of 2:1 as detailed in Table 5.1. Biostate contains human albumin as a stabiliser.
The FVIII/vWF complex in Biostate is purified from cryoprecipitate using selective precipitation and chromatography steps. The manufacturing process of Biostate includes solvent detergent, dry heat treatment, and partitioning steps for inactivation/removal of HIV, hepatitis A, hepatitis B, and hepatitis C and also have some effect on parvovirus B19.
Table 5.1: Biostate 1000 IU FVIII / 2000 IU VWF Composition, final volume 10 mL per vial.
Indications for Use
Biostate is indicated for:
- Prophylaxis and treatment of non-surgical and surgical bleeding in patients with von Willebrand disease when desmopressin (DDAVP) treatment is ineffective or contraindicated
- Prophylaxis and treatment of non-surgical and surgical bleeding associated with FVIII deficiency due to haemophilia A
The number of vials of Biostate to be reconstituted is determined by dividing the total dose of FVIII or vWF:RCo required by the number of IU of that ingredient in the selected vial size. This should be rounded up to the nearest whole number of vials.
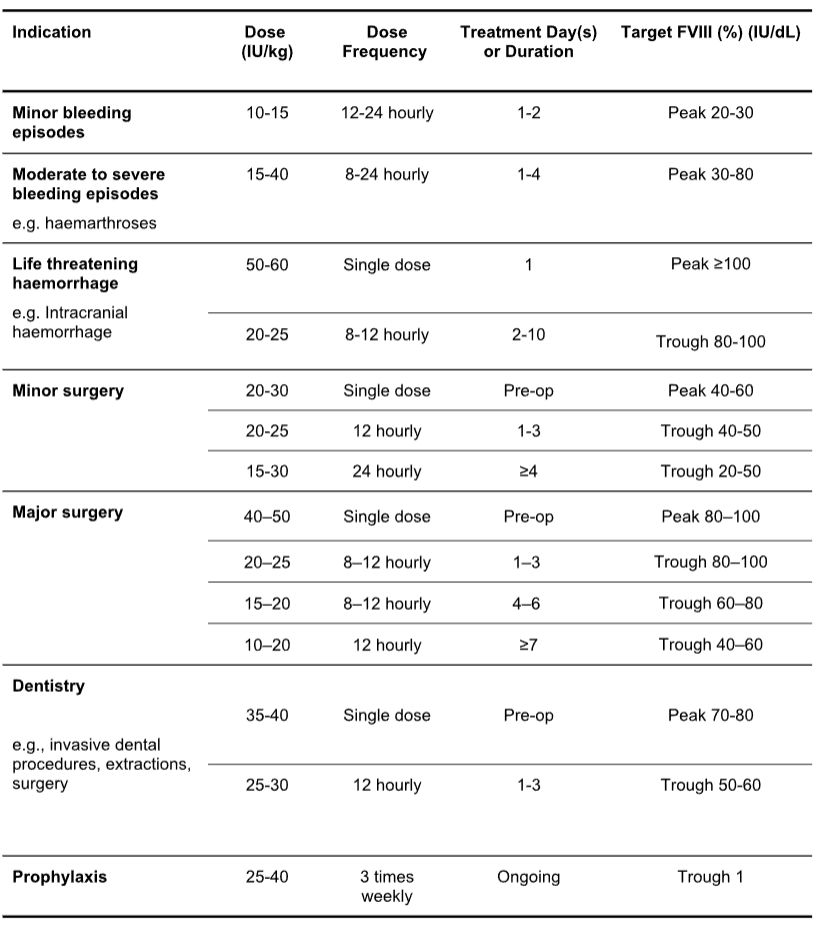
Table 5.2: Biostate Dosage Guidelines for Haemophilia A
Table 5.3: Biostate Dosage Guidelines for Von Willebrand Disease1
- Allergic reactions
Allergic reactions or fever are rarely observed. Depending on the nature of an adverse reaction, the rate of injection should be slowed or stopped to alleviate symptoms. - Antibodies to factor VIII
Patients with congenital factor VIII deficiency may develop neutralising alloantibodies (inhibitors) to factor VIII after treatment. If this occurs specialist advice must be sought. - Antibodies to von Willebrand factor
Patients with von Willebrand disease, especially type 3 patients, may very rarely develop neutralising alloantibodies (inhibitors) to von Willebrand factor. Such antibodies may occur in close association with anaphylactic reactions. Therefore, patients experiencing anaphylactic reactions should be evaluated for the presence of an inhibitor. - Thrombosis
Thromboembolic events have rarely been reported in vWD patients receiving coagulation factor replacement therapy, especially in the setting of known risk factors for thrombosis and may be related to the generation of supranormal FVIII levels. Those with aquired vWD are also potentially at risk.