Transfusion medicine
Transfusion medicine handbook
The Transfusion Medicine Handbook is designed to assist hospital staff and other health professionals in modern Transfusion Medicine Practice.
- About NZBS
- Abbreviations and Glossary
- Foreword
- 1. Introduction
-
2. Collection, Testing and Processing of Blood Donation
- 2.1 Blood donors
- 2.2 Donor Selection Criteria
- 2.3 Self-sufficiency and the Volunteer Status of Donors
- 2.4 Informed Consent for Donation
- 2.5 Apheresis Donation
- 2.6 Directed and Designated Donation
- 2.7 Haemochromatosis
- 2.8 Cord Blood Donors
- 2.9 Testing of Donor Blood
- 2.10 Leucodepletion
- 2.11 Processing of Collected Blood to Components
- 2.12 Processing of Collected Blood to Fractionated Products
- 2.13 Blood Components and Fractionated Products as Medicines
-
3. Guide to Good Transfusion Practice
- Overview
- 3.1 Clinical Governance
- 3.2 Prescribing Blood Components and Fractionated Products
- 3.3 Informed Consent to Receive a Blood Transfusion
- 3.4 Requesting Blood Components and Fractionated Products
- 3.5 Blood Stock Management: the Maximum Blood Order Schedule
- 3.6 Collecting Blood Samples for Pre-transfusion Testing
- 3.7 Pretransfusion Testing
- 3.8 Patients With a Positive Antibody Screen
- 3.9 Sample Validity (‘72-hour Rule’)
- 3.10 Provision of Red Cells in an Emergency
- 3.11 Removal From Storage and Time Limits for Transfusion
- 3.12 Administration and Observation of Transfusion
- 3.13 Rate of Transfusion and Precautions
- 3.14 Electromechanical Infusion Pumps
- 3.15 Blood Administration Sets and Filters
- 3.16 Warming of Blood Components
- 3.17 Compatible Intravenous Solutions
- 3.18 Adding Medication to Blood Components
- 3.19 Documentation of Transfusion
- 3.20 Local Systems and Procedures
- 3.21 Reporting of Adverse Events
-
4. Blood Components
- Overview
- 4.1 ABO Blood Groups and Antibodies
- 4.2 Avoiding ABO Incompatible Transfusions
- 4.3 RhD Antigen
- 4.4 Other Blood Group Systems
- 4.5 Cytomegalovirus (CMV)
- 4.6 Irradiation
- 4.7 Blood Components Available From NZBS
- 4.8 Red Cell Components
- 4.9 Platelet Components
- 4.10 Granulocyte Components
- 4.11 Plasma Components
- 4.12 Fresh Frozen Plasma
- 4.13 Cryoprecipitate Apheresis-high Fibrinogen
- References
-
5. Fractionated Products
- Overview and General Administration Guide
- 5.1 Coagulation Factors
- 5.1.1 BIOSTATE (Factor VIII / vWF)
- 5.1.2 PROTHROMBINEX-VF (Factors II, IX and X)
- 5.1.3 BERIPLEX® NZ (Factors II, VII, IX, X and Protein C & S)
- 5.1.4 FEIBA NF (Factor VIII inhibitor bypassing fraction)
- 5.1.5 RiaSTAP (Fibrinogen)
- 5.1.6 FIBROGAMMIN (Factor XIII)
- 5.2 Natural Inhibitors of Coagulation
- 5.2.1 KYBERNIN P
- 5.3 Albumin Solutions
- 5.3.1 ALBUREX® 5 NZ (Human albumin 5%)
- 5.3.2 ALBUMEX 4 (Human albumin 4%)
- 5.3.3 ALBUREX 20 NZ (Human albumin 20%)
- 5.3.4 ALBUMEX 20 (Human albumin 20%)
- 5.4 Immunoglobulin Preparations
- 5.4.1 NORMAL IMMUNOGLOBULIN-VF
- 5.4.2 HEPATITIS B IMMUNOGLOBULIN-VF
- 5.4.3 HyperHEP B
- 5.4.4 TETANUS IMMUNOGLOBULIN-VF
- 5.4.5 TETAGAM P
- 5.4.6 ZOSTER IMMUNOGLOBULIN-VF
- 5.4.7 BERIRAB P (Rabies Immunoglobulin)
- 5.4.8 Rh(D) Immunoglobulin-VF (Anti-D immunoglobulin)
- 5.4.9 RHOPHYLAC (Anti-D immunoglobulin)
- 5.4.10 PRIVIGEN NZ / PRIVIGEN (Normal Immunoglobulin) 10% intravenous immunoglobulin (IVIg)
- 5.4.11 GAMUNEX 10%
- 5.4.12 HIZENTRA NZ / HIZENTRA
- 5.5 Other Products
- 5.5.1 BERINERT (C1-esterase inhibitor)
- 5.5.2 BERINERT SC (C1-esterase inhibitor; subcutaneous administration)
- 5.6 Products from Australian Lifeblood (ARC Lifeblood)
- References
-
6. Special Circumstances
- 6.1 Management of Acute Blood Loss
- 6.2 Massive Haemorrhage Pathway
- 6.3 Indications for MHP Activation
- 6.4 Code Crimson/ Code Red/ Trauma Pathway
- 6.5 Adult Massive Haemorrhage Pathway Algorithm
- 6.6 Standard MHP Pathway
- 6.7 Obstetric MHP Pathway
- 6.8 Complications of Acute Blood Loss Associated with Large Volume Transfusions
- 6.9 Avoidable Haemostatic Problems in Elective Surgery
- 6.10 Oral Anticoagulant Induced Bleeding Or Overdose
- 6.11 Thrombolytic Therapy
- 6.12 Disseminated Intravascular Coagulation (DIC)
- 6.13 Cardiopulmonary Bypass
- 6.14 Haemolytic Disease of the Fetus and Newborn (HDFN)
- 6.15 Intrauterine Transfusion (IUT)
- 6.16 Transfusion of the Newborn
- 6.17 Special Requirements
- 6.18 Neonatal Autoimmune Thrombocytopenia
- 6.19 Fetal and Neonatal Alloimmune Thrombocytopenia (FNAIT)
- 6.20 Individuals Refusing Blood Transfusion
- References
-
7. Adverse Effects of Transfusion
- 7.1 Overview
- 7.2 Reporting Adverse Reactions and Events
- 7.3 Guidelines for the Management of Adverse Transfusion Reactions (111I015)
- 7.4 Febrile Non-haemolytic Transfusion Reaction
- 7.5 Allergic & Anaphylactic Reaction
- 7.6 Transfusion-associated Hypotension
- 7.7 Acute Haemolytic Transfusion Reaction
- 7.8 Delayed Haemolytic Transfusion Reaction
- 7.9 Bacterial Sepsis
- 7.10 Post-transfusion Purpura
- 7.11 Transfusion-associated Circulatory Overload
- 7.12 Transfusion-related Acute Lung Injury
- 7.13 Transfusion-associated Dyspnoea
- 7.14 Transfusion-associated Graft-versus-host Disease
- 7.15 Iron Overload / Haemosiderosis
- 7.16 Transfusion-related Immunosuppression
- 7.17 Transfusion-transmitted Infection
- 7.18 Other Infectious Agents
- 7.19 Adverse Event Data
- 7.20 Other Complications
- 8. Clinical Alternatives and Applications
-
9. Other Services Provided by NZBS
- 9.1 Therapeutic Apheresis
- 9.2 Therapeutic Venesection
- 9.3 Cellular Therapy Services
- 9.4 Tissue Bank
- 9.5 Serum Eye Drops
- 9.6 Red Cell Reference Laboratory (Immunohaematology)
- 9.7 New Zealand Transplantation And Immunogenetics Laboratory (NZTIL)
- 9.8 New Zealand Bone Marrow Donor Registry (NZBMDR)
- 9.9 Organ Donation New Zealand
- 9.10 Setting Up New Transfusion Facilities
- 10. NZBS Sample Requirements
4. Blood Components
4.6 Irradiation
Transfusion-associated graft-versus-host-disease (TA-GvHD) is a rare but usually fatal complication of transfusion. The risk associated with an individual transfusion depends on the number and viability of contaminating lymphocytes, the susceptibility of the patient's immune system to donor lymphocyte engraftment (primarily related to the degree of T-cell immunosuppression) and the degree of immunological incompatibility between donor and patient.
Cellular components, with the exception of thawed haematopoietic progenitor cells (HPC) intended for transplantation, must be irradiated to prevent TA-GvHD in at-risk patients. It should be noted that all platelet units in New Zealand are irradiated routinely. Frozen plasma components and fractionated products do not require irradiation.
The following tables 4.2 to 4.5 summarise the clinical indications for blood component irradiation according to the Australian and New Zealand Society for Blood Transfusion, and the British Society for Haematology [1] [2]. ANZSBT have flowcharts which can be helpful in decisions about irradiation [1].
It should be noted that these are tables are based on guidelines and individual cases may warrant their own assessment of risk and benefit.
Table 4.2: Definite Clinical Indications for Use of Irradiated Blood Components [2]
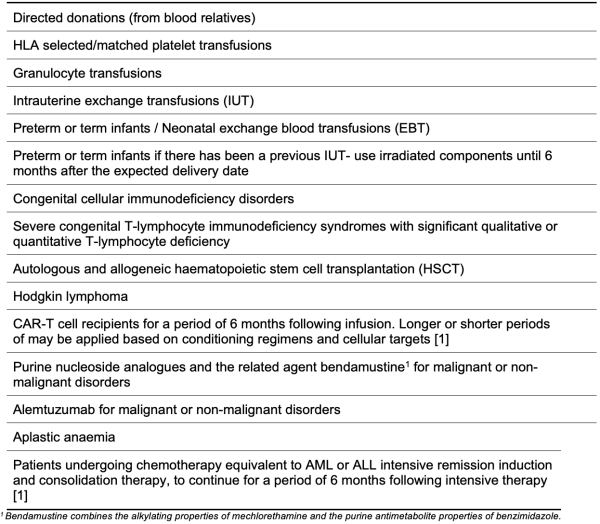
Table 4.3: Possible Clinical Indications for Use of Irradiated Blood Components
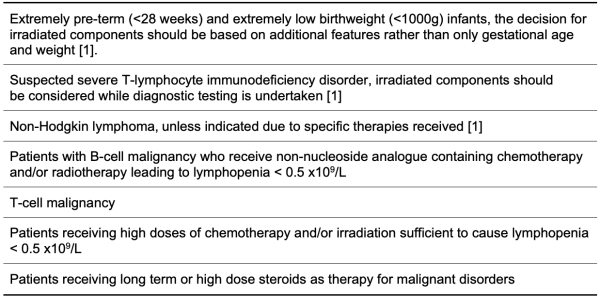
Table 4.4: No Clinical Indication for Use of Irradiated Blood Components
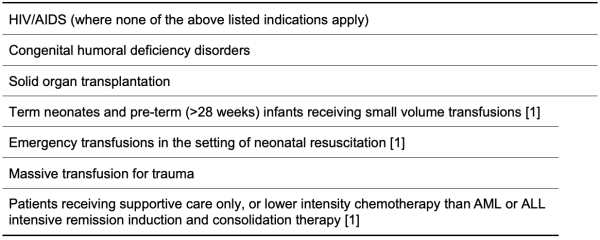
Table 4.5: Recommendations on Duration of Use of Irradiated Blood Components [2]