Transfusion medicine
Transfusion medicine handbook
The Transfusion Medicine Handbook is designed to assist hospital staff and other health professionals in modern Transfusion Medicine Practice.
- About NZBS
- Abbreviations and Glossary
- Foreword
- 1. Introduction
-
2. Collection, Testing and Processing of Blood Donation
- 2.1 Blood donors
- 2.2 Donor Selection Criteria
- 2.3 Self-sufficiency and the Volunteer Status of Donors
- 2.4 Informed Consent for Donation
- 2.5 Apheresis Donation
- 2.6 Directed and Designated Donation
- 2.7 Haemochromatosis
- 2.8 Cord Blood Donors
- 2.9 Testing of Donor Blood
- 2.10 Leucodepletion
- 2.11 Processing of Collected Blood to Components
- 2.12 Processing of Collected Blood to Fractionated Products
- 2.13 Blood Components and Fractionated Products as Medicines
-
3. Guide to Good Transfusion Practice
- Overview
- 3.1 Clinical Governance
- 3.2 Prescribing Blood Components and Fractionated Products
- 3.3 Informed Consent to Receive a Blood Transfusion
- 3.4 Requesting Blood Components and Fractionated Products
- 3.5 Blood Stock Management: the Maximum Blood Order Schedule
- 3.6 Collecting Blood Samples for Pre-transfusion Testing
- 3.7 Pretransfusion Testing
- 3.8 Patients With a Positive Antibody Screen
- 3.9 Sample Validity (‘72-hour Rule’)
- 3.10 Provision of Red Cells in an Emergency
- 3.11 Removal From Storage and Time Limits for Transfusion
- 3.12 Administration and Observation of Transfusion
- 3.13 Rate of Transfusion and Precautions
- 3.14 Electromechanical Infusion Pumps
- 3.15 Blood Administration Sets and Filters
- 3.16 Warming of Blood Components
- 3.17 Compatible Intravenous Solutions
- 3.18 Adding Medication to Blood Components
- 3.19 Documentation of Transfusion
- 3.20 Local Systems and Procedures
- 3.21 Reporting of Adverse Events
-
4. Blood Components
- Overview
- 4.1 ABO Blood Groups and Antibodies
- 4.2 Avoiding ABO Incompatible Transfusions
- 4.3 RhD Antigen
- 4.4 Other Blood Group Systems
- 4.5 Cytomegalovirus (CMV)
- 4.6 Irradiation
- 4.7 Blood Components Available From NZBS
- 4.8 Red Cell Components
- 4.9 Platelet Components
- 4.10 Granulocyte Components
- 4.11 Plasma Components
- 4.12 Fresh Frozen Plasma
- 4.13 Cryoprecipitate Apheresis-high Fibrinogen
- References
-
5. Fractionated Products
- Overview and General Administration Guide
- 5.1 Coagulation Factors
- 5.1.1 BIOSTATE (Factor VIII / vWF)
- 5.1.2 PROTHROMBINEX-VF (Factors II, IX and X)
- 5.1.3 BERIPLEX NZ (Factors II, VII, IX, X and Protein C & S)
- 5.1.4 FEIBA NF (Factor VIII inhibitor bypassing fraction)
- 5.1.5 RiaSTAP (Fibrinogen)
- 5.1.6 FIBROGAMMIN (Factor XIII)
- 5.2 Natural Inhibitors of Coagulation
- 5.2.1 KYBERNIN P
- 5.3 Albumin Solutions
- 5.3.1 ALBUREX 5 NZ (Human albumin 5%)
- 5.3.2 ALBUMEX 4 (Human albumin 4%)
- 5.3.3 ALBUREX 20 NZ (Human albumin 20%)
- 5.3.4 ALBUMEX 20 (Human albumin 20%)
- 5.4 Immunoglobulin Preparations
- 5.4.1 NORMAL IMMUNOGLOBULIN-VF
- 5.4.2 GamaSTAN
- 5.4.3 HEPATITIS B IMMUNOGLOBULIN-VF
- 5.4.4 HyperHEP B
- 5.4.5 TETANUS IMMUNOGLOBULIN-VF
- 5.4.6 TETAGAM P
- 5.4.7 ZOSTER IMMUNOGLOBULIN-VF
- 5.4.8 BERIRAB P (Rabies Immunoglobulin)
- 5.4.9 Rh(D) Immunoglobulin-VF (Anti-D immunoglobulin)
- 5.4.10 RHOPHYLAC (Anti-D immunoglobulin)
- 5.4.11 PRIVIGEN NZ / PRIVIGEN (Normal Immunoglobulin) 10% intravenous immunoglobulin (IVIg)
- 5.4.12 GAMUNEX 10%
- 5.4.13 HIZENTRA NZ / HIZENTRA
- 5.5 Other Products
- 5.5.1 BERINERT (C1-esterase inhibitor)
- 5.5.2 BERINERT SC (C1-esterase inhibitor; subcutaneous administration)
- 5.6 Products from Australian Lifeblood (ARC Lifeblood)
- 5.7 References
-
6. Special Circumstances
- 6.1 Management of Acute Blood Loss
- 6.2 Massive Haemorrhage Pathway
- 6.3 Indications for MHP Activation
- 6.4 Code Crimson/ Code Red/ Trauma Pathway
- 6.5 Adult Massive Haemorrhage Pathway Algorithm
- 6.6 Standard MHP Pathway
- 6.7 Obstetric MHP Pathway
- 6.8 Complications of Acute Blood Loss Associated with Large Volume Transfusions
- 6.9 Avoidable Haemostatic Problems in Elective Surgery
- 6.10 Oral Anticoagulant Induced Bleeding Or Overdose
- 6.11 Thrombolytic Therapy
- 6.12 Disseminated Intravascular Coagulation (DIC)
- 6.13 Cardiopulmonary Bypass
- 6.14 Haemolytic Disease of the Fetus and Newborn (HDFN)
- 6.15 Intrauterine Transfusion (IUT)
- 6.16 Transfusion of the Newborn
- 6.17 Special Requirements
- 6.18 Neonatal Autoimmune Thrombocytopenia
- 6.19 Fetal and Neonatal Alloimmune Thrombocytopenia (FNAIT)
- 6.20 Individuals Refusing Blood Transfusion
- References
-
7. Adverse Effects of Transfusion
- 7.1 Overview
- 7.2 Reporting Adverse Reactions and Events
- 7.3 Guidelines for the Management of Adverse Transfusion Reactions (111I015)
- 7.4 Febrile Non-haemolytic Transfusion Reaction
- 7.5 Allergic & Anaphylactic Reaction
- 7.6 Transfusion-associated Hypotension
- 7.7 Acute Haemolytic Transfusion Reaction
- 7.8 Delayed Haemolytic Transfusion Reaction
- 7.9 Bacterial Sepsis
- 7.10 Post-transfusion Purpura
- 7.11 Transfusion-associated Circulatory Overload
- 7.12 Transfusion-related Acute Lung Injury
- 7.13 Transfusion-associated Dyspnoea
- 7.14 Transfusion-associated Graft-versus-host Disease
- 7.15 Iron Overload / Haemosiderosis
- 7.16 Transfusion-related Immunosuppression
- 7.17 Transfusion-transmitted Infection
- 7.18 Other Infectious Agents
- 7.19 Adverse Event Data
- 7.20 Other Complications
- 8. Clinical Alternatives and Applications
-
9. Other Services Provided by NZBS
- 9.1 Therapeutic Apheresis
- 9.2 Therapeutic Venesection
- 9.3 Cellular Therapy Services
- 9.4 Tissue Bank
- 9.5 Serum Eye Drops
- 9.6 Red Cell Reference Laboratory (Immunohaematology)
- 9.7 New Zealand Transplantation And Immunogenetics Laboratory (NZTIL)
- 9.8 New Zealand Bone Marrow Donor Registry (NZBMDR)
- 9.9 Organ Donation New Zealand
- 9.10 Setting Up New Transfusion Facilities
- 10. NZBS Sample Requirements
5. Fractionated Products
5.4.2 GamaSTAN
GamaSTAN is a sterile, preservative-free solution containing 16.5% protein (mainly as human immune globulin) made from overseas donations [9]. GAMASTAN is provided in 10 mL vials and is intended for intramuscular injection. Do not administer GAMASTAN intravenously.
It is supplied by NZBS under Section 29, a provision in the Medicines Act 1981, whereby a medical practitioner can prescribe a medicine that is not registered with Medsafe.
Indications for Use
Table 5.11: Clinical Indications for Use of GAMASTAN [9] 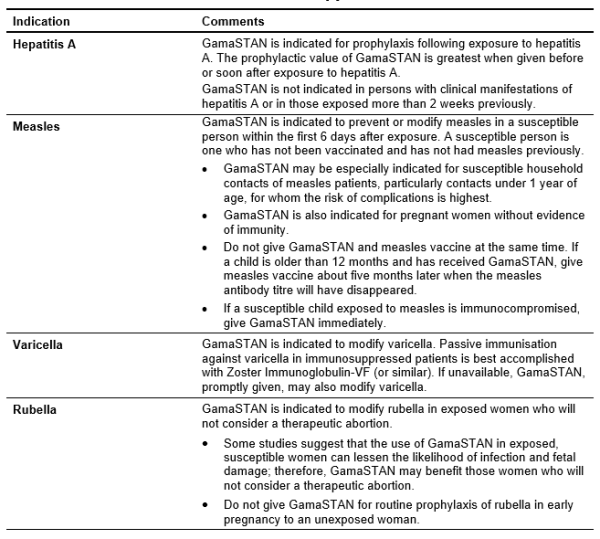
GamaSTAN is not indicated for routine prophylaxis or treatment of viral hepatitis type B, rubella, poliomyelitis, mumps or varicella.
Dosage and Administration
Table 5.12: Dosage Recommendations for GamaSTAN [9] [10]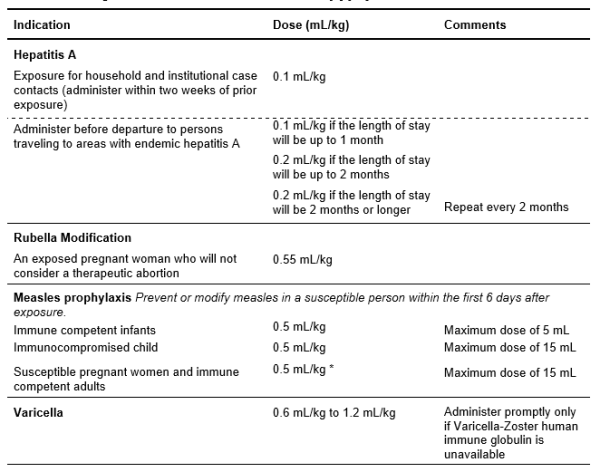 *Datasheet specifies 0.25 mL/kg for susceptible people; however, the NZBS’ Clinical Advisory Group (July 2025) recommends 0.5 mL/kg due to declining measles antibody levels in populations with widespread immunisation.
*Datasheet specifies 0.25 mL/kg for susceptible people; however, the NZBS’ Clinical Advisory Group (July 2025) recommends 0.5 mL/kg due to declining measles antibody levels in populations with widespread immunisation.
Defer live vaccine administration for up to six (6) months.
Observe the recipient for 20 minutes after administration.
